Zinc Sulphate
Zinc sulphate is obtained through zinc ash, zinc carbonate, or zinc oxide treatment with sulphuric acid. Pharmaceutical-grade zinc sulphate is usually used high purity zinc oxide as a raw material. As for industrial uses, zinc sulphate is commonly available as anhydrous or heptahydrate forms. We provide zinc sulphate in different grades, quantities, and packaging for your market and industry solution.

- Zinc Sulphate
- Zinc Sulphate Monohydrate
- Zinc Sulfate Heptahydrate
Overview
IUPAC Name : Zinc sulfate
Chemical Formula : ZnSO4
Cas No. : 7733-02-0
Appearance : White crystalline powder or granular
HS Code : 2833.26.10
Zinc Sulfide (ZnS) is a naturally occurring salt that appears in liquid form as a yellowish-white powder. It has two common crystalline forms, which are Sphalerite (zinc blende) and Wurtzite. Sphalerite has a cubic crystal structure, and this form is the predominant form in nature. Meanwhile, wurtzite has a hexagonal crystal which is made by heating the sphalerite to 1020°C. Zinc sulfide is commonly employed as a pigment for paint, plastic, and rubber. It is also phosphorescent, which makes it useful for several decorative and electronic applications, such as luminescent cosmetics, x-ray, glowing paint, etc.
Manufacturing Process
Zinc sulfide is obtained as by-product from the synthesis of ammonia from methane. The hydrogen sulfide impurities in the natural gas reacts with the zinc oxide to form zinc sulfide.
ZnO + H2S → ZnS + H2O
Another method is through the reaction of zinc sulfate and sodium sulfide, followed by calcination. The zinc sulfide is produced as the hydrogen sulfide passes the zinc salt formed from the initial reaction. Elemental zinc and sulfur can also be reacted together to form zinc sulfide. However this is a violent reaction and is accompanied by rapid evolution of gas, light, and heat.
Application
Paint Industry
It can be used as a white pigment for paint, linoleum, or synthetic leather.
Medical Industry
Zinc sulfide is used as a luminiscent material or phosphor for cathode ray tubes, X-ray screens and other glow-in-the-dark products. The zinc sulfide can be used as pigment to dental rubber.
Semiconductor Industry
Both the polymorphs of zinc are intrinsic, wide-bandgap semiconductors. They are identified as prototypical II-VI semiconductors, and they adopt structures related to many of the other semiconductors, such as gallium arsenide. The sphalerite has a band gap of about 3.54 electron volts at 300 kelvins, while the wurtzite has a band gap of about 3.91 electron volts. It can be doped as either an n-type semiconductor or a p-type semiconductor.
Other Industries
Zinc sulfide can be used as an infrared optical material and an efficient photocatalyst. It is also used as phosphor in television screens and watches. Due to its versatility as a white pigment it can also be used in manufacture of cosmetics.
Overview
IUPAC Name : Zinc Sulphate Monohydrate
Chemical Formula : ZnSO4.H2O
Cas No. : 7446-19-7
Appearance : White crystalline powder or granular
HS Code : 2833.29.30
Zinc sulphate monohydrate is historically known as white vitriol. It is an inorganic compound that is commonly used as a feed
additive for livestock. It also has other applications, including zinc supplement for human, water purification, fiber industry, and beer brewing. Zinc sulphate occurs in three types of hydrate. Zinc sulphate is listed on the World Health Organization’s List of Essential Medicines. The monohydrate exists as the gunningite mineral in nature.
Manufacturing Process
Water/wash water is filled in the Digester and Sulphuric acid is slowly added to the digester, while the quantity of Zinc ash is added slowly with agitation. The addition is continued until the completion of the reaction. As the reaction is complete, the suspension is drained in the filtration tank for storage. The suspension is filtered with the filter press. The residue is washed and filtered again for better recovery. Then the filtrate is fed to the crystallizer. The cooling is provided by circulating chilled water from chilling plant. The solution is stirred continuously. As the required temperature is reached the Zinc Sulphate crystals separate out and the separated crystals are centrifuged with the help of centrifuge to remove any liquor remaining. For Zinc Sulphate Monohydrate, the filtrate is dried in a spray drying system which is collected in a hopper and packed in bags to be sent to the market.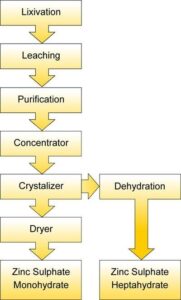
Application
Zinc sulfate monohydrate is a readily-accessible form of zinc supplementation and is commonly used as an additive for livestock feed. This helps prevent zinc deficiencies in animals, which can lead to issues such as impaired reproduction, hair loss, reduced feed intake and growth, and skin lesions.
- Animal Feed
Zinc Sulphate Monohydrate can be an additive for the livestock feed to serve as a supplement for zinc. Zinc deficiency in livestock may lead to horrific issues in the livestock, such as hair loss, skin lesion, impaired production, and reduced feed intake and growth.
According to the regulation of the feeding stuffs set by the EU, the safe intake of zinc sulphate monohydrate for the water in drinking are:
- Milk replacers: 20 mg/L
- Swine: 30 mg/L
- Poultry 40 mg/L
- Daily intake for cattle: 500 mg
- Daily intake for dairy cows: 2000 mg
- Other Application
Other applications for Zinc Sulphate Monohydrate are including, but not limited to:
- Zinc supplement for human that suffers zinc
- Natal supplementation for pregnant woman to help with natal health and reduce the risk of delivering a preterm
- Oral rehydration therapy (ORT) for individuals who have suffered serious dehydration due to Consuming Zinc Sulphate allows the body to absorb more water and prevents critical dehydration.
- It can be used to control and inhibit moss growth on roofs, often as a component of roof cleaning soaps and anti-moss/antifungal products.
- Zinc sulphate is also commonly used as a supplement during the beer brewing process. It is used to pushes the yeast to the limits of fermentation and requires proper nutrition to ensure consistent.
Overview
IUPAC Name : Zinc Sulfate Heptahydrate
Chemical Formula : ZnSO4.7H2O
Cas No. : 7446-20-0
Appearance : White crystalline powder or granular
HS Code : 28332990
Doctors prescribe zinc sulphate hydrates as part of oral rehydration therapy. They use it to treat diarrhoea or stomach issues related to zinc deficiency. Some people use it as a dietary supplement, and doctors also use it in intravenous feeding.
| PHYSICAL & CHEMICAL INFORMATION | |
|---|---|
| Physical State; Appearance GRANULES OR CRYSTALLINE POWDER. Physical dangers Chemical dangers | Formula: ZnSO4. 7H2O Molecular mass: 287.6 Melting point: 100°C Density: 1.97 g/cm³ Solubility in water, g/100ml at 20°C: 54 |
Manufacturing Process
Zinc sulfate crystallises from aqueous solution as a heptahydrate, zinc sulfate-7-water, ZnSO4. 7H2O. You can make zinc sulfate-7-water in the laboratory by reacting zinc carbonate with dilute sulfuric acid.



